Since launching the CODA study just over a year ago:

1,076 patients have been screened
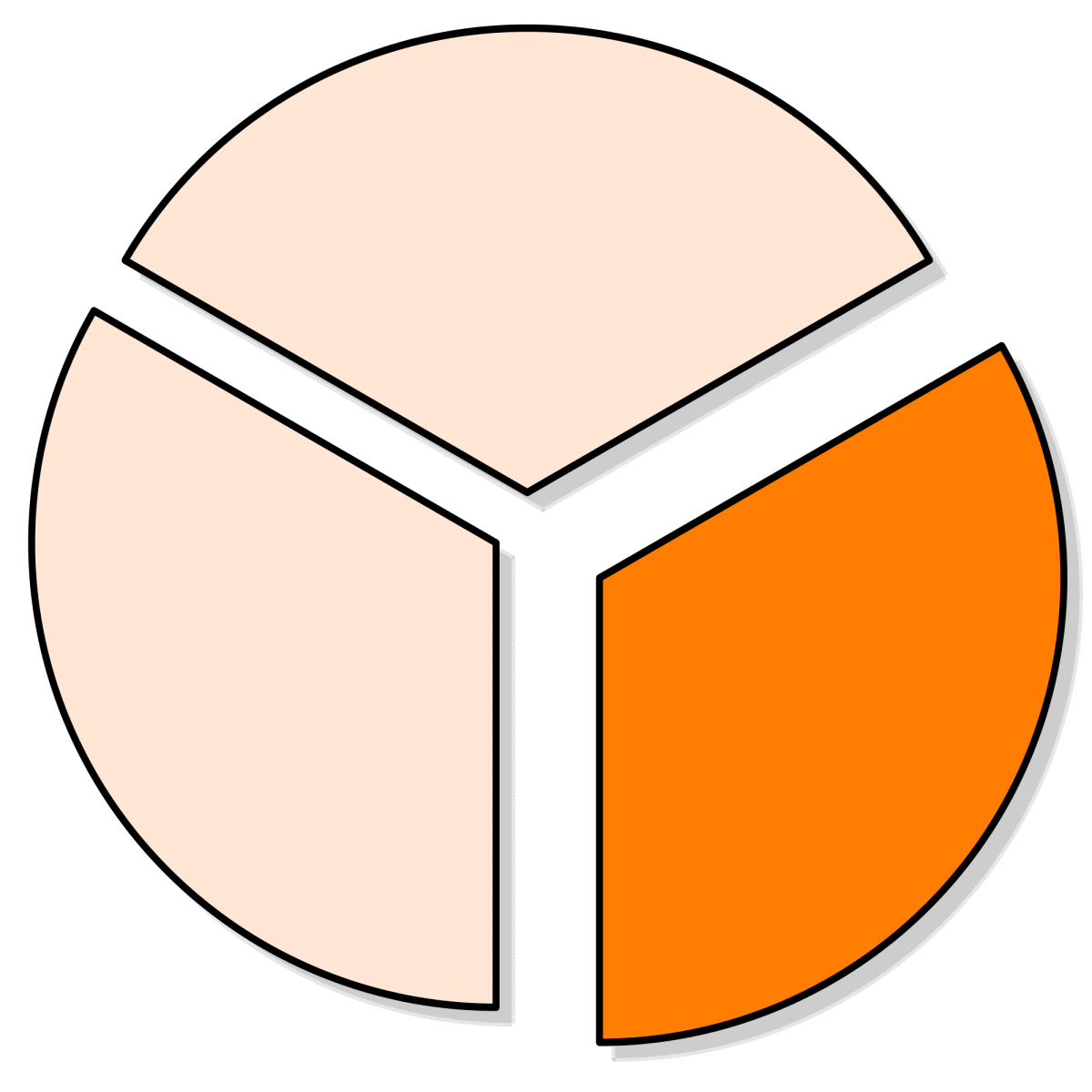
Approximately 1/3 of eligible patients enrolled in the randomized cohort

Patient retention is at 91% for the randomized cohort
It’s been an exciting spring with continued steady enrollment. We are also happy to announce that NYU’s Tisch Hospital and NYC Health + Hospitals/Bellevue, Beth Israel Deaconess Medical Center, and Boston Medical Center will be joining the CODA study with an anticipated summer launch, which will bring us to 12 sites! Expanding to other sites will enable us to hit our enrollment goals, and we’re excited to expand across the country to access a more diverse population.
Led by Dr. Giana Davidson, we are also applying for an ancillary study grant. Ancillary studies are meant to build on existing research studies and provide extra funds to answer different research questions on the same topic.
We have a unique opportunity to improve our ability to predict which patients are more likely to be successfully treated with antibiotics by measuring specific proteins in the blood that tell us about the degree of inflammation. These extra lab tests could help patients and healthcare providers make more informed treatment decisions.We hypothesize that patients with more extreme inflammation at the time they come forward for initial examination and diagnosis will be more likely to fail management with antibiotics.
To measure these proteins, we would ask patients enrolled in the randomized and observational groups of the CODA study to donate a small amount (about a tablespoon) of blood. This blood would be drawn either from their previously placed IV line (requiring no additional needle stick), or it may require an additional blood draw. Participants would be given $25 for their time, and no additional follow-up would be required outside of the CODA study.
We will keep you informed about this trial as we go through the application process with National Institutes of Health.


